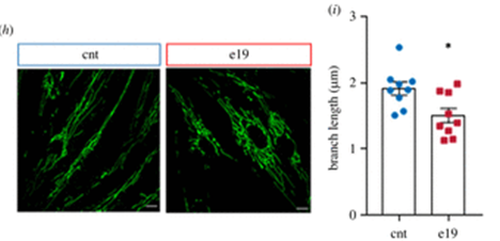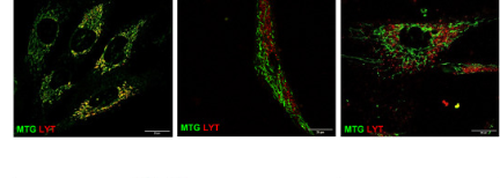
Fig. 1 Confocal microscopy assessment of mitophagy in control and mutant LIG3 fibroblasts. Panels represent merged images of CNT, 1-1, and 3-2, costained with MitoTracker Green and LysoTracker Red. Three visual fields in at least 3 independent samples per fibroblast type were analyzed. Scale bars: 20 μm.
Research themes
The biochemistry of metabolism and bioenergetics study the chemical processes that occur within cells to produce and utilize energy. Metabolism encompasses a set of biochemical reactions that convert nutrients into energy and fundamental molecules for life. Bioenergetics focuses on the mechanisms by which cells generate and store energy, primarily through mitochondrial oxidative phosphorylation and ATP production. Our research group is dedicated to studying the metabolic and bioenergetic processes, with the aim of gaining a deeper understanding of their molecular mechanisms and translating this knowledge into clinical applications for human health. The group’s work is structured around three main research themes:
1. Understanding Metabolic and Bioenergetic Mechanisms
Metabolic and bioenergetic processes are highly regulated to ensure energy balance and adaptation to environmental changes. A deeper understanding of these processes is crucial for elucidating the molecular basis of metabolic diseases, aging, neurodegenerative disorders, and cancer. Our studies focus on:
- Analyzing metabolic pathways and the interactions between metabolism and cellular signaling under both physiological and pathological conditions.
- Investigating the role of mitochondrial bioenergetics in the regulation of cellular homeostasis.
- Examining cellular metabolism in diseases such as diabetes, obesity, cancer, and neurodegeneration.
2. Study of the Effects of Plant-Derived Bioactive Compounds on Metabolism and Cellular Bioenergetics
Plant-derived bioactive molecules can modulate key biological processes such as metabolism, bioenergetics, inflammation, and oxidative stress, making them a field of growing interest in medicine and nutraceuticals. Understanding the molecular basis of their function is essential for the prevention and treatment of various diseases. Our research group aims to:
- Analyze the impact of plant compounds on the regulation of carbohydrate, lipid, and protein metabolism.
- Investigate the influence of these substances on ATP production, mitochondrial dynamics, and oxidative stress management.